Product Selection
For which Roche product do you have a complaint?
Do you suspect a product falsification?
Product Information
Please provide as many details about your product as possible. Refer to the diagram on the right side to find where the requested information is typically found.
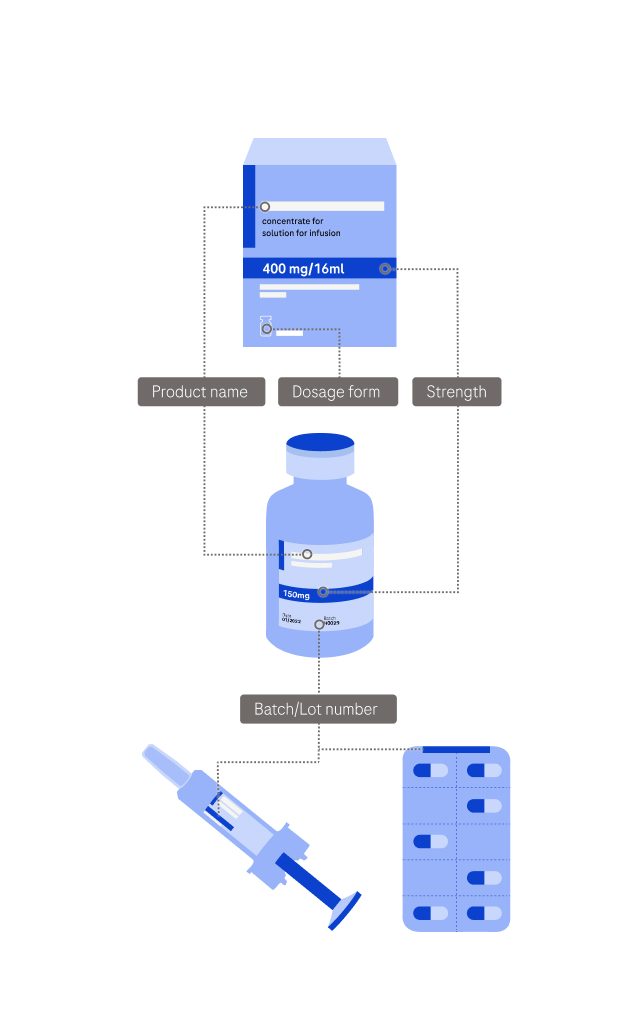
Product selected
Please select the procedure to which your concern relates:
Please select your product type (dosage form).
Is your product available for return?
Complaint Details
Please describe the defect in as much detail as you can. If possible, please also upload a photo of the product and defect.
Examples of potential product deficiencies may include (but are not limited to):
- Empty blister cavity
- Size/shape variation
- Unusual appearance
- Foreign matter
- Cracked cap
- Counterfeit product
- Reconstitution issues
- Discoloration
- Missing label
- Component not working
- Missing component
- Missing lot number or expiration date
How is it different from "normal"? Please add a detailed description of the defect (e.g. appearance, damage, leakage).
Describe at what point was the defect or issue discovered (e.g. when opening the box, before the administration, after the administration, at which handling step according to instructions of use or patient information leaflet)
Photo Upload
Please support the investigation process by attaching multiple photos of the defect(s) and its packaging from all sides.
Please support the investigation process by attaching multiple photos of the defect(s) and its packaging from all sides.
Max files: 15
Max overall size 20 MB
Permissible file types: .png, .gif, .jpg, .bmp, .pdf
Max files: 15
Max overall size 20 MB
Permissible file types: .png, .gif, .jpg, .bmp, .pdf
Consideration for photos
What to photograph
Take photos of: 1. Defect(s) with all relevant angles, 2. product with all sides, 3. packaging with all sides. Ensure product details like product strength, expiry date and batch / lot number are clearly visible.
How to take the photographs
Ensure the photos are well-lit and sharp.
Consideration for photos
What to photograph
Take photos from: The product displaying the defect - from all relevant angles, containing all relevant details.
How to take the photographs
Ensure the photos are well-lit and sharp.
Complaint Details Continued
Please answer the following questions to the best of your knowledge.
How was the product stored?
How long have you/patient/caregiver been using the product?
Was the product administered at home or in a medical environment (e.g. doctors office)?
By whom?
Please provide the date of complaint occurrence:
Was there a delay in administration of the product as a result of this complaint?
Did the administrator of the product receive any handling training?
Has this complaint also been reported via an additional channel (e.g. Health Authority / Call center/ Pharmacy) ?
Have there been any side effects involved?
Personal Details
Are you a…
If permitted by local regulations, do you want to be informed about the outcome of the complaint investigation?
F. Hoffmann-La Roche Ltd ("Roche") has legal obligations to record and/or report product complaints. Therefore, for such purposes your data will be processed in accordance with specific drug safety legislation, as further described in the Roche Privacy Notice for Pharmacovigilance, medical information and product complaints.
Roche will keep a record of the personal data that you provide for the purpose of responding to your inquiry, to follow up on such requests, to maintain the information in the Roche product complaint database for reference and to comply with our legal and regulatory recording and reporting obligations. The retention time of data is in accordance with respective laws and regulations.
By including personal data of another data subject in the form, you represent the lawfulness of processing of such personal data.
The Privacy Policy provides further detailed information about your rights and how Roche processes personal data.