Examples of potential side effects / adverse events include:
- Headache
- Nausea (feeling sick)
- Vomiting
Please select your country from the list below to visit your local Roche Medical Information site. If your country is not available, please exit to return to the Global experience.
An adverse event (AE) is any untoward medical occurrence in a patient or clinical trial subject administered a medicinal product and which does not necessarily have a causal relationship with this treatment. An adverse event (AE) can therefore be any unfavorable and unintended sign, symptom or disease temporarily associated with the use of a medicinal product, whether or not considered related to the medicinal product.
Miguel is attending a regional medical conference. One of the speakers at the conference is a patient who is receiving a Roche medicine for her condition. During the presentation, the patient tells the audience that when she first started taking the medicine she experienced frequent headaches. Because of this, her doctor reduced his dosage and after that, her headaches went away.
Miguel must report this potential side effect because the new information may impact the safety profile of the medicine.
Please confirm your Country of residence / practice on order to route your request to the responsible locale team.
当您报告不良事件和个人信息时,请注意以下要求:
姓名请提供姓名首字母缩写
工作单位请勿提供军警院校、党政军或涉密单位的信息
请勿提供具体的地址信息
此外,请勿提供任何与生日,民族相关的个人信息
Please note the following requirements when reporting side effects and personal information:
For the name, please provide initials only.
Do not provide information from military, police institutions, party or government offices, or confidential units as your workplace.
Do not provide specific address information.
Additionally, do not provide any personal information related to birthdays or ethnicity.
An Adverse Event (AE), also known as a side effect, is an unwanted and unintended response in a patient, after receiving a medicinal product, that may or may not have been caused by treatment with this product. This includes all events where harm or unintended and unwanted response in a patient, user or other person as a result of the use of a medical device. The information you provide helps to ensure the safety of our products and our patients.
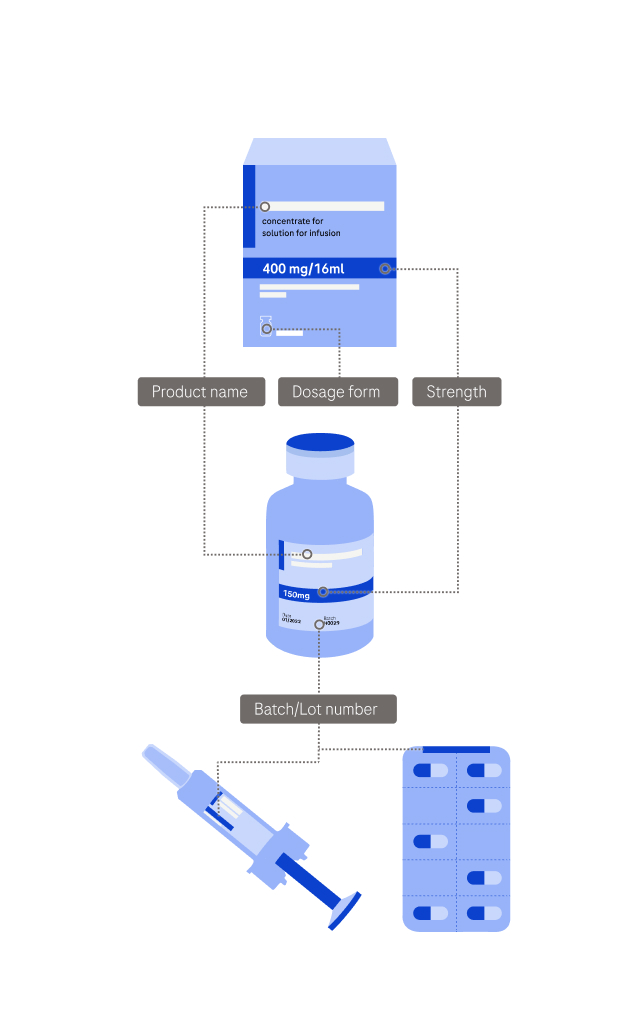
Side effects may include (but are not limited to) symptoms like:
Please describe the adverse event as accurate as possible and provide as many details as possible, like for example: The [side effect] occured after taking [the product] on [date]. The [side effect] started on [date] and the patient was admitted into hospital. The [side effect] stopped on [date].
The pregnancy-related questions are meant to collect information about side effects and pregnancy. Thank you for contributing to making our products safer and more efficient.
Describe here the side effect, if this has resolved or is ongoing, how serious this is/was, the outcome and any further information that may be relevant both before, during and after the side effect occurred. For medical devices please also describe the way the device caused or contributed to the event and if relevant clearly describe the device malfunction causing the event.
Information about the use of our medicines during pregnancy is valuable to us to increase knowledge of their safety and inform patients. Please note, patients should consult with their healthcare professional to be informed on the safety of taking a medicine during pregnancy.
To improve the safety of our medication, any information (at least 1 identifying detail) you can provide is very helpful. The data will be stored in accordance with data privacy regulations.
If you consent in contacting your healthcare professional, please provide details below.
F. Hoffmann-La Roche Ltd ("Roche") has legal obligations to record and/or report adverse events. Therefore, for such purposes your data will be processed in accordance with specific GVP (pharmacovigilance) legislation, as further described in the Roche Privacy Notice for Pharmacovigilance, medical information and product complaints.
Roche will keep a record of the personal data that you provide for the purpose of responding to your inquiry, to follow up on such requests, to maintain the information in the Roche global pharmacovigilance database for reference and to comply with our legal and regulatory recording and reporting obligations. The retention time of data is in accordance with respective laws and regulations.
By including personal data of another data subject in the form, you represent the lawfulness of processing of such personal data.
The Privacy Policy provides further detailed information about your rights and how Roche processes personal data.
当您报告不良事件和个人信息时,请注意以下要求:
姓名请提供姓名首字母缩写
工作单位请勿提供军警院校、党政军或涉密单位的信息
请勿提供具体的地址信息
此外,请勿提供任何与生日,民族相关的个人信息
Please note the following requirements when reporting side effects and personal information:
For the name, please provide initials only.
Do not provide information from military, police institutions, party or government offices, or confidential units as your workplace.
Do not provide specific address information.
Additionally, do not provide any personal information related to birthdays or ethnicity.
By following this link, you are leaving Medical Information and entering a website that is not owned or controlled by Roche. Roche does not take any responsibility for access to or use of this website, nor for any content therein.