Who is contacting us?
A temperature excursion or breach is defined as storage or exposure of a Roche product outside the labelled storage temperatures for any length of time.
Are you a…
Temperature Excursion Details
Definition:
A temperature excursion or breach is defined as storage or exposure of a Roche product outside the labelled storage temperatures for any length of time.
Please provide details of the temperature excursion but do not provide any personal data (including sensitive personal data). To enable an accurate assessment, it is important that the time and temperature data you have provided are both complete and accurate. You will be able to add the affected products on the next page.
Specify recorded temperature(s) excursion (one value is mandatory)
Duration below lower limit
Duration above upper limit
How many times did the temperature drop below 0.5ºC and came back up to 0.5ºC?
Any other details?
Attach your fridge log sheet (optional)
Product Information
Additional products? ({{form.products[index].index}})
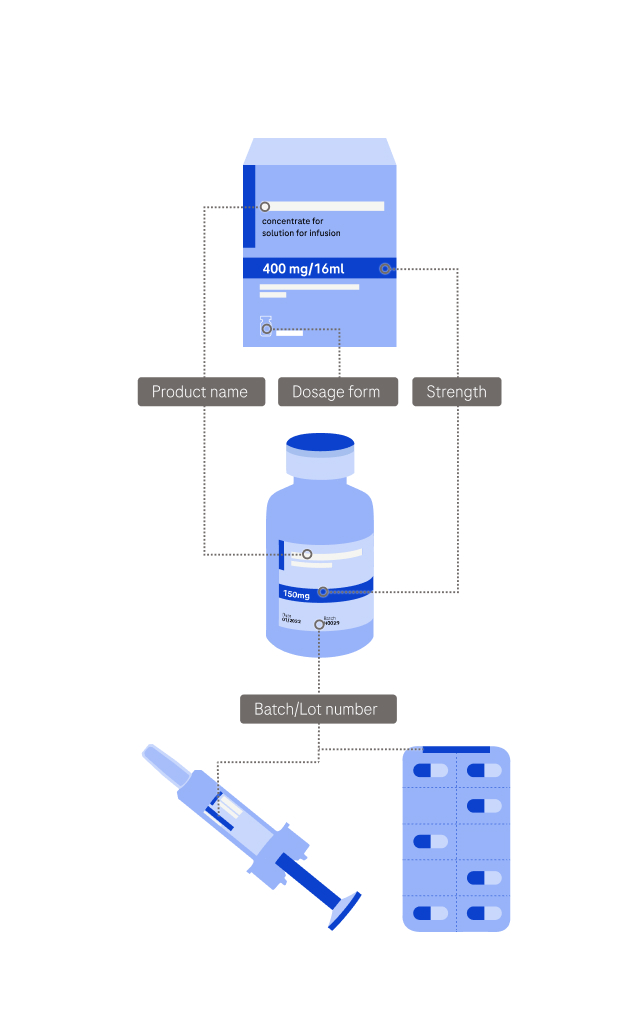
Please provide information on the affected products. You can add multiple products by using the “Add Product” button at bottom of this page.
Concerning Columvi (Glofitamab), Enspryng (satralizumab), Itovebi (inavolisib), Lunsumio (mosunetuzumab), Piasky (Crovalimab), Ronapreve (casirivimab & imdevimab), Rozlytrek (entrectinib), Vabysmo (faricimab):
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
If a pregnancy occurs while using Herceptin/PERJETA/Kadcyla/Phesgo or within 7 months following the last dose of Herceptin/Kadcyla/Phesgo or within 6 months following the last dose of PERJETA, please immediately report pregnancy to the local Roche Adverse Event line at +32 (0)2 525 82 99.
Additional information will be requested during a Herceptin/PERJETA//Phesgo/Kadcyla-exposed pregnancy and the first year of the infant's life. This will enable Roche to better understand the safety of Herceptin/PERJETA/Kadcyla/Phesgo and to provide appropriate information to health authorities, healthcare providers and patients.
Which products were affected in the reported excursion?
Do you have a patient waiting for the product?
Has the product been involved in a previous temperature excursion while in your possession?
Has the product been reconstituted/diluted?
Is the product at its final storage location before being administered /dispensed /supplied to the patient?
Has the product been administered to the patient?
Products affected by the reported excursion
Contact Details
Please complete the form by adding your contact details
I consent to my data being processed for the purpose of responding to my inquiry and in accordance with the Roche Privacy Policy & Privacy Notice for Pharmacovigilance.
You consent, by ticking the box above, to the processing of your data for the purposes of responding to your inquiry and in accordance with the Roche Privacy Notice for Pharmacovigilance, medical information and product complaints. If you do not consent to the data processing, we will not be able to reply to your message and kindly ask you to use other channels to contact us.
F. Hoffmann-La Roche Ltd ("Roche") will keep a record of the personal data that you provide for the purpose of responding to your inquiry, to follow up on such requests, to maintain the information in the Roche medical information database for reference and to comply with our legal and regulatory recording and reporting obligations. The retention time of data is in accordance with respective laws and regulations.
By including personal data of another data subject in the form, you represent the lawfulness of processing of such personal data.
The Roche Privacy Policy provides further detailed information about your rights and how Roche processes personal data.
Your report

Which Roche product has been affected? Please provide as much information as possible about the product and event but do not provide any personal data (including sensitive personal data). This information will be sent to the local Roche office who may contact you for further information. You will receive a notification once your case has been assessed. Please ensure that product is correctly stored while waiting to hear back from us.
Concerning Columvi (Glofitamab), Enspryng (satralizumab), Itovebi (inavolisib), Lunsumio (mosunetuzumab), Piasky (Crovalimab), Ronapreve (casirivimab & imdevimab), Rozlytrek (entrectinib), Vabysmo (faricimab):
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
If a pregnancy occurs while using Herceptin/PERJETA/Kadcyla/Phesgo or within 7 months following the last dose of Herceptin/Kadcyla/Phesgo or within 6 months following the last dose of PERJETA, please immediately report pregnancy to the local Roche Adverse Event line at +32 (0)2 525 82 99.
Additional information will be requested during a Herceptin/PERJETA//Phesgo/Kadcyla-exposed pregnancy and the first year of the infant's life. This will enable Roche to better understand the safety of Herceptin/PERJETA/Kadcyla/Phesgo and to provide appropriate information to health authorities, healthcare providers and patients.
Product name
Additional Product Details
What happened to the product?
Contact Details
Please complete the form by adding your contact details
I consent to my data being processed for the purpose of responding to my inquiry and in accordance with the Roche Privacy Policy & Privacy Notice for Pharmacovigilance.
You consent, by ticking the box above, to the processing of your data for the purposes of responding to your inquiry and in accordance with the Roche Privacy Notice for Pharmacovigilance, medical information and product complaints. If you do not consent to the data processing, we will not be able to reply to your message and kindly ask you to use other channels to contact us.
F. Hoffmann-La Roche Ltd ("Roche") will keep a record of the personal data that you provide for the purpose of responding to your inquiry, to follow up on such requests, to maintain the information in the Roche medical information database for reference and to comply with our legal and regulatory recording and reporting obligations. The retention time of data is in accordance with respective laws and regulations.
By including personal data of another data subject in the form, you represent the lawfulness of processing of such personal data.
The Roche Privacy Policy provides further detailed information about your rights and how Roche processes personal data.